Photomicrographs
On this page, we have posted photographs of various hydrothermal minerals and textures that we have taken over the years. We will add to these photographs and aim to post something new each month. Click on any photograph to see a larger image.
Actinolite Hydrothermal amphiboles (actinolite-tremolite) form at high temperature (>290°C) in near-neutral pH conditions, and usually where permeability is low.


Adularia Typically, vein adularia crystals are very small and difficult to see within quartz because of their similar relief and birefringence (both are slightly lower in adularia). Here you can see three large rhomb shaped adularia crystals with patchy twinning, and if you look carefully, a few smaller grains in the quartz. Vein adularia indicates neutral pH and good permeability, and can form over a wide range of temperatures, from about 150 to 300°C. It is often associated with boiling fluids, and hence with gold in epithermal vein deposits.

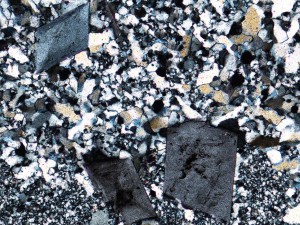
Alunite, an aluminium sulphate mineral, is diagnostic of acid fluids, and so is often found with minerals like kaolinite and diaspore. There is a solid solution between Na and K end members, with natroalunite predominating at low temperatures. At very low temperatures, alunite forms very small crystals with psuedocubic outlines, but thin randomly oriented plates like those below are more typical.


Anhydrite forms in a range of environments, including permeable zones where there is boiling, mixing of acid and neutral fluids, from magmatic SO2, and from heating of seawater. It can form over a wide temperature range, but at low temperature it readily alters to gypsum.

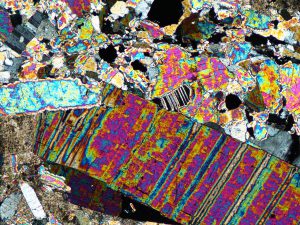
Arsenopyrite, an iron arsenic sulphide, is commonly associated with pyrite, and occurs in many gold deposits. It can form over a wide temperature range.


Azurite and malachite are hydrous copper carbonates that generally form in low temperature, oxidised, low pH environments, often as a weathering product above copper-bearing sulphide deposits.


Barite is common in submarine geothermal systems, and in their fossil equivalents, VHMS (volcanic hosted massive sulphide) deposits, where it can be accompanied by gold and other metals, including copper, lead, zinc and silver. It also occurs in some high and low-sulphidation epithermal deposits, and Carlin-type deposits. Barite can form over a very wide temperature range, and so can be found with low and high-temperature minerals.


Bornite is an important copper ore mineral which is deposited at high temperatures, particularly in porphyry and skarn deposits.

Calcite has many crystal forms, but one of the most spectacular is the platy form which is deposited from boiling fluids. It is therefore found beneath the surface in active geothermal systems, and may be preserved in some low sulphidation epithermal veins (as here), although more commonly the calcite plates are replaced by quartz as the system cools, with quartz preserving the platy texture.


Chalcedony is a cryptocrystalline form of silica (SiO2), composed of very fine radiating intergrowths of quartz (which has a trigonal structure) and moganite (which is monoclinic). Chalcedony forms where silica deposition is very rapid, and generally at less than 190°C, though rarely it can form at higher temperatures.


Chlorite encompasses a group of minerals that are common in geothermal systems, especially where temperatures are moderate (<300°C), pH is near neutral, and permeability is moderate to low.


Covellite is present in many high sulphidation gold deposits, but also commonly forms during the supergene alteration of rocks containing copper


Cristobalite is a silica mineral that forms metastably from low temperature (<200°C), often slightly acid hydrothermal fluids, and also through deuteric alteration.


Diaspore (AlO(OH)) is indicative of acid fluids where it has formed by hydrothermal alteration (as in this case). However, it can also form by weathering (in bauxites) and metamorphism of aluminous rocks.


Dickite is a higher temperature polymorph of kaolinite, which forms in the presence of near neutral to acid fluids at temperatures of about 200 to 250°C.


Enargite forms at moderate to high temperatures, under relatively oxidising, acid conditions. It commonly occurs with quartz, alunite, covellite and luzonite in advanced argillic assemblages, and is diagnostic of high-sulphidation epithermal mineralisation.


Epidote is a key temperature indicator mineral in geothermal systems, as it only forms above about 240°C, or higher in the case of euhedral crystals like these. It varies from almost colourless to very yellow in thin section, and is normally clearly visible in rock samples.
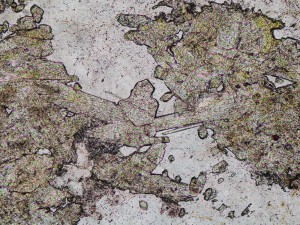

Fluorite can occur in zones of acid hydrothermal alteration, and is common in some skarns and greisens.
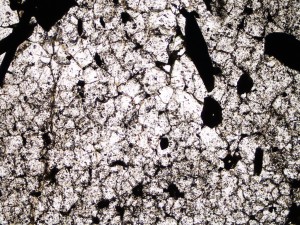

Garnet The garnet that occurs in geothermal systems is usually hydrogrossular or andradite, and can be weakly anisotropic and zoned. It forms at high temperatures (>280°C), and so is often accompanied by minerals such as actinolite and biotite.
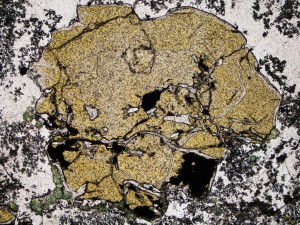

Gypsum forms at low temperatures (<120°C) in geothermal systems, and often replaces anhydrite. It indicates a high sulphate environment, which may be acid or oxidising.


Illite-Smectite At temperatures between about 150 and 230°C and in the presence of near neutral to slightly acid fluids, alteration is often dominated by interlayered illite-smectite clays, with the percentage of illite interlayering increasing with temperature. XRD analysis is the best method to verify the presence and amount of interlayering.


Illite with no interlayered smectite forms at temperatures above 230°C in active geothermal systems where there are near neutral to slightly acid fluids. It is often accompanied by quartz and chlorite.

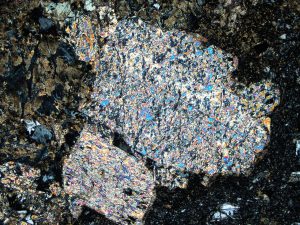
Jarosite is an iron sulphate that forms by weathering or low temperature hydrothermal alteration of pyrite and other sulphides, often in the presence of acid fluids. It can resemble epidote where it is well crystalline, though it is often very fine and granular. There is a sodium variety (natro-jarosite) that is almost identical, and best distinguished with XRD analysis.

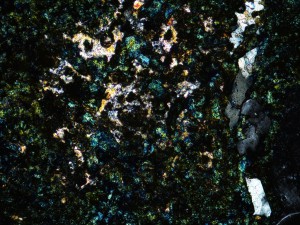
Kaolinite is a clay that usually forms in acid conditions, but can also form from cool (less than about 220°C) low salinity waters with near-neutral pH, or during weathering. Good XRD analysis is required to discriminate between kaolinite and the other kandite polymorphs (dickite, nacrite), which form at higher temperatures. Kaolinite is common in late-stage hydrothermal overprints, where it may be accompanied by carbonates.


Laumontite, a calcium zeolite, forms under low temperature (<220°C), neutral pH hydrothermal conditions, generally where fCO2 is low. In this case, fCO2 must have increased later, so that calcite was deposited between the laumontite crystals. Laumontite also forms in low grade regional and thermal metamorphism.


Luzonite is a polymorph of enargite, and like it, forms in acid conditions, generally in high-sulphidation epithermal gold deposits. It can be difficult to distinguish from enargite, but twinning should be visible within crystals.


Magnetite generally forms at temperatures over 300°C in hydrothermal systems, though it is also a primary mineral in many volcanic and plutonic rocks. It is common in gold-rich porphyry deposits, some skarns, and iron oxide copper gold deposits.

Natrolite, a sodium zeolite, forms under low temperature (usually <150°C), neutral pH hydrothermal conditions, so occurs in the shallow parts of some hydrothermal and epithermal systems. XRD analysis is required for positive identification.
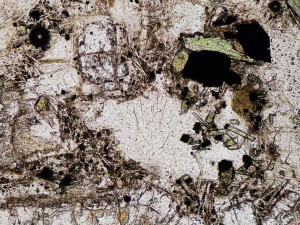

Opal is an amorphous, hydrous silica mineraloid (not a true mineral because it lacks a crystal structure) that forms where temperatures are low (<120°C), silica contents are high, and often, though not always, where pH is low (acid). It forms at very shallow levels in geothermal systems, including in sinters and scales within wells and pipelines, and over time gradually reverts to quartz.


Prehnite: hydrothermal prehnite is most common in basic volcanics and calcareous sediments, and usually in areas of low permeability, though it can be found in veins and depositing inside geothermal wells. It generally indicates temperatures above 210°C, and fluids of near neutral pH.


Pumpellyite: like prehnite, hydrothermal pumpellyite is most common in mafic volcanics where permeability is low. It generally forms in neutral pH fluids at temperatures above 270°C, and is associated with minerals like chlorite, epidote, prehnite and wairakite.


Framboidal Pyrite: Pyrite is common in many mineral deposits, but framboidal pyrite is much less common. This form of pyrite rarely occurs in some hydrothermal deposits, including quartz-rich epithermal veins, as in this example. The conditions that lead to the deposition of pyrite framboids are uncertain, but include a low temperature reducing environment, and possibly a magnetic precursor mineral.
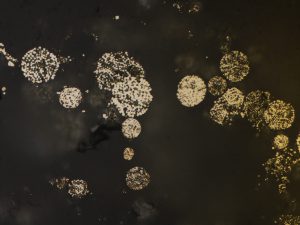
Pyrophyllite indicates acid (pH<4) fluids, and usually high temperatures (>220°, especially where diaspore and dickite/nacrite or quartz are also present), though in places it coexists with opal (i.e. low T). It is present in some high sulphidation epithermal deposits, and in porphyry lithocaps.

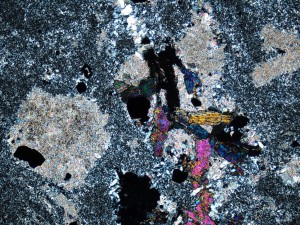
Quartz forms under a very wide range of conditions, and is stable in all but very acid fluids. The closely spaced banding in this vein indicates rapidly fluctuating conditions, which is typical of epithermal veins.


Realgar (and orpiment) forms at low temperatures from arsenic-rich fluids. The melting point is 307°C, but it is generally deposited at less than 200°C, and sometimes <100°C in hot springs.
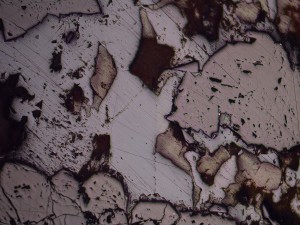

Rhodochrosite is a manganese carbonate that is usually pink in hand specimen, and occurs in some epithermal and orogenic gold deposits. Manganese minerals are commonly regarded as indicative of intermediate sulphidation epithermal deposits, but this example is from the Martha Mine, Waihi (New Zealand), which is generally regarded as a type example of low sulphidation mineralisation. Like other carbonates, rhodochrosite can form over a wide temperature range and generally near neutral to slightly acid conditions.


Scorodite is usually of supergene origin, and forms in an oxidising environment where arsenic is commmon.

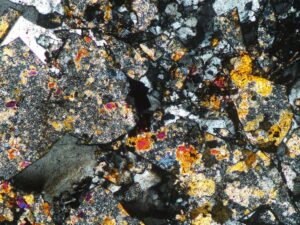
Stibnite is a shiny grey metallic antimony mineral that is often present in shallow levels of epithermal systems. Crystals are soft, and often twinned and bent.


Sulphur generally indicates cool, acid, and oxidising conditions, where it can form by the oxidation of H2S, or reduction of SO2. But liquid sulphur has also been observed at higher temperatures in geothermal and oil wells above its melting point (113°C).
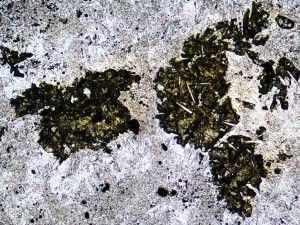
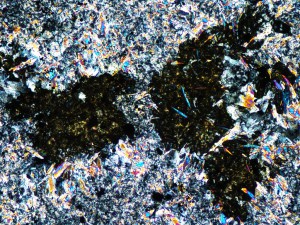
Talc can form at high temperature in hydrothermal systems, where it coexists with minerals like actinolite, but can also form at low temperature due to weathering. In this instance, smectite suggests that it formed at low temperature.


Tourmaline generally deposits from hydrothermal solutions where there is a strong magmatic influence, and therefore in the deeper and hotter (>250°C) parts of geothermal systems.

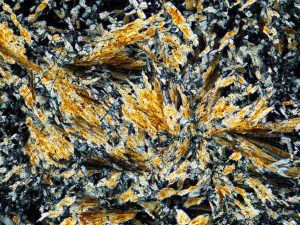
Wairakite forms at temperatures above 200°C, in fluids of near neutral pH, and low CO2. Vein crystals often indicate boiling.


Zoisite forms under similar conditions to epidote, but is more common in contact and regional metamorphic rocks, and in skarns than in geothermal systems.

